Transformations in Matter and Energy Carbon TIME is an NSF-funded partnership led by Michigan State University
Systems and Scale
Systems and Scale is the first of the six Carbon TIME units. If you are new to teaching Carbon TIME, read the Carbon TIME FAQ: Which Units Should I Teach? .
The goal of the Systems and Scale unit is to introduce students to organic matter and chemical energy (in the context of combustion) using the tools for reasoning and environmental literacy practices that students will engage with in other units. Students develop required capacity to distinguish organic matter from inorganic matter, and to understand how differences in the chemical make-up of materials influences how materials and energy are transformed and moved between systems.
The Systems and Scale Unit supports students in using core disciplinary ideas, science practices, and cross-cutting concepts to develop scientific explanations of how matter and energy are transformed during combustion of different organic materials.
Follow these steps to get ready to teach the Systems and Scale Unit
 |
 |
 |
 |
 |
Lead Editor for 2019 Version
Kirsten D. Edwards, Department of Teacher Education, Michigan State University
Principal Authors
Hannah K. Miller, Education Department, Johnson State University
Jenny Dauer, School of Natural Resources, University of Nebraska-Lincoln
Christa Haverly, Department of Teacher Education, Michigan State University
Kirsten D. Edwards, Department of Teacher Edwards, Michigan State University
Christie Morrison Thomas, Department of Teacher Education, Michigan State University
Charles W. “Andy” Anderson, Department of Teacher Education, Michigan State University
Contributing Authors
Elizabeth Xeng de los Santos, Beth Covitt, Jennifer Doherty, Allison Freed, Wendy Johnson, Deborah Jordan, Craig Kohn, Lindsey Mohan, Joyce Parker, Elizabeth Tompkins, Nick Verbanic
Illustrations
Craig Douglas, Kendra Mojica
This research is supported in part by grants from the National Science Foundation: A Learning Progression-based System for Promoting Understanding of Carbon-transforming Processes (DRL 1020187) and Sustaining Responsive and Rigorous Teaching Based on Carbon TIME (NSF 1440988). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the united States Department of Energy.
Contact the MSU Environmental Literacy Program for more information: EnvLit@msu.edu.
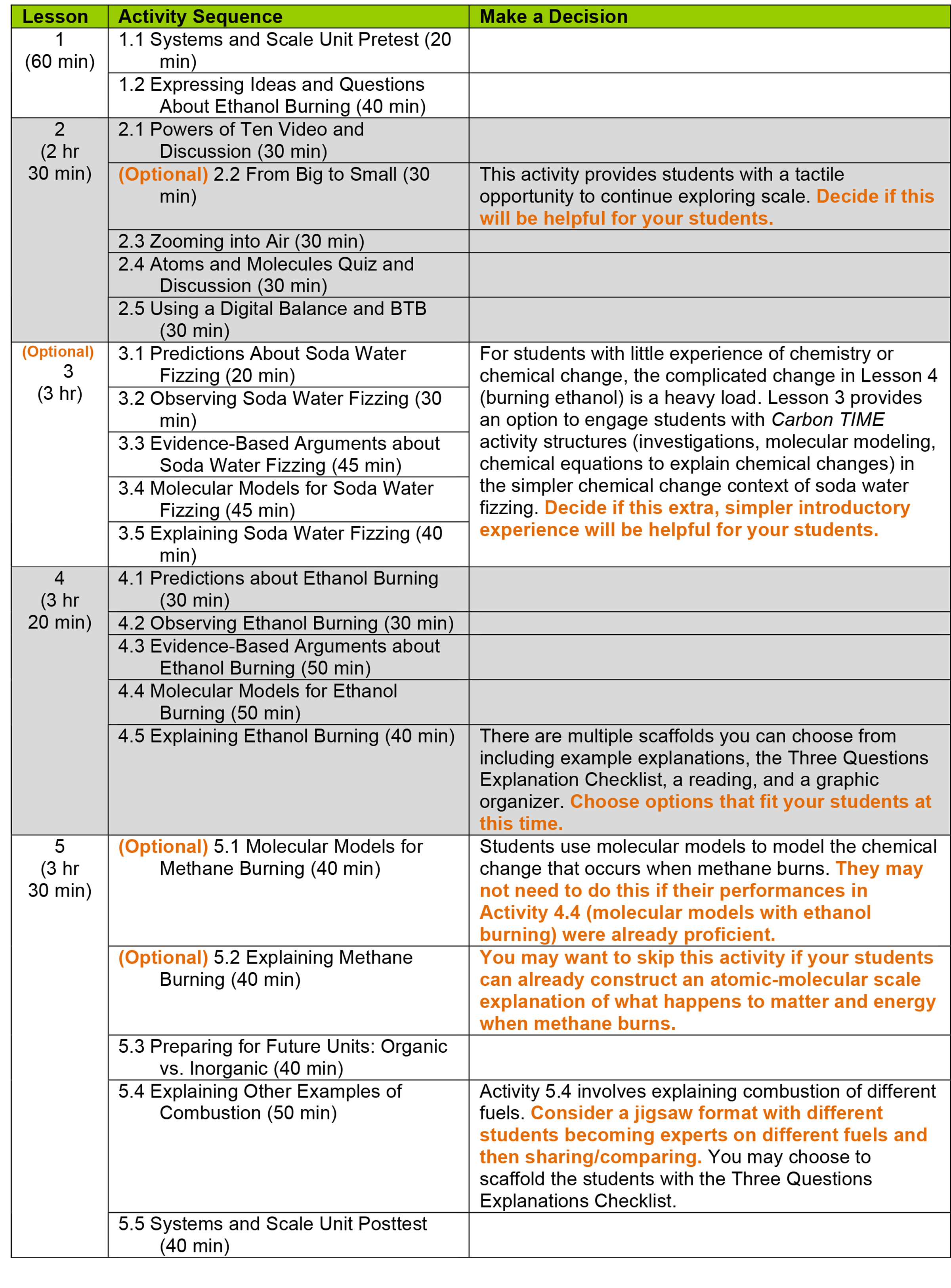
Before beginning the Systems and Scale Unit, you need to decide what to teach and importantly, what not to teach! Use this page to choose the unit sequence that’s most appropriate for your students.
- Optional activities in the Systems and Scale Unit are designed to provide supportive learning opportunities for students who have not previously studied or who are still working to develop proficiency with regard to reasoning about scale and chemical change. Use your professional judgment to decide whether or not to teach each optional lesson or activity.
Unless otherwise noted in the table below, all activities in the unit should be taught.
Systems and Scale is the first of the six Carbon TIME units. If you are new to teaching Carbon TIME, read the Carbon TIME FAQ: Which Units Should I Teach? .
The goal of the Systems and Scale unit is to introduce students to organic matter and chemical energy (in the context of combustion) using the tools for reasoning and environmental literacy practices that students will engage with in other units. Students develop required capacity to distinguish organic matter from inorganic matter, and to understand how differences in the chemical make-up of materials influences how materials and energy are transformed and moved between systems.
The Systems and Scale Unit supports students in using core disciplinary ideas, science practices, and cross-cutting concepts to develop scientific explanations of how matter and energy are transformed during combustion of different organic materials.
Follow these steps to get ready to teach the Systems and Scale Unit
 |
 |
 |
 |
 |
Lead Editor for 2019 Version
Kirsten D. Edwards, Department of Teacher Education, Michigan State University
Principal Authors
Hannah K. Miller, Education Department, Johnson State University
Jenny Dauer, School of Natural Resources, University of Nebraska-Lincoln
Christa Haverly, Department of Teacher Education, Michigan State University
Kirsten D. Edwards, Department of Teacher Edwards, Michigan State University
Christie Morrison Thomas, Department of Teacher Education, Michigan State University
Charles W. “Andy” Anderson, Department of Teacher Education, Michigan State University
Contributing Authors
Elizabeth Xeng de los Santos, Beth Covitt, Jennifer Doherty, Allison Freed, Wendy Johnson, Deborah Jordan, Craig Kohn, Lindsey Mohan, Joyce Parker, Elizabeth Tompkins, Nick Verbanic
Illustrations
Craig Douglas, Kendra Mojica
This research is supported in part by grants from the National Science Foundation: A Learning Progression-based System for Promoting Understanding of Carbon-transforming Processes (DRL 1020187) and Sustaining Responsive and Rigorous Teaching Based on Carbon TIME (NSF 1440988). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the united States Department of Energy.
Contact the MSU Environmental Literacy Program for more information: EnvLit@msu.edu.
Before beginning the Systems and Scale Unit, you need to decide what to teach and importantly, what not to teach! Use this page to choose the unit sequence that’s most appropriate for your students.
- Optional activities in the Systems and Scale Unit are designed to provide supportive learning opportunities for students who have not previously studied or who are still working to develop proficiency with regard to reasoning about scale and chemical change. Use your professional judgment to decide whether or not to teach each optional lesson or activity.
Unless otherwise noted in the table below, all activities in the unit should be taught.

 Download PDF Unit Front Matter
Download PDF Unit Front Matter