Transformations in Matter and Energy Carbon TIME is an NSF-funded partnership led by Michigan State University
(Optional) Lesson 3 - Investigating and Explaining Soda Water Fizzing
Overview
Students investigate changes in mass and CO2 concentration for soda water fizzing. Then they explain results using molecular models and chemical equations to answer the Movement Question and the Matter Change Question.
Guiding Question
What happens when soda water loses its fizz?
Activities in this Lesson
Note: Lesson 3 is optional depending on
your knowledge of your students and learning goals. This lesson is recommended for middle schoolers to introduce them to using investigations, molecular models, and chemical equations to describe a simple chemical change. See the Systems and Scale Unit Read Me file for more information to consider when making this choice.
- Activity 3.1: Predictions about Soda Water Fizzing (20 min)
- Activity 3.2: Observing Soda Water Fizzing (30 min)
- Activity 3.3: Evidence-Based Arguments about Soda Water Fizzing (45 min)
- Activity 3.4: Molecular Models for Soda Water Fizzing (45 min)
- Activity 3.5: Explaining Soda Water Fizzing (40 min)
Unit Map
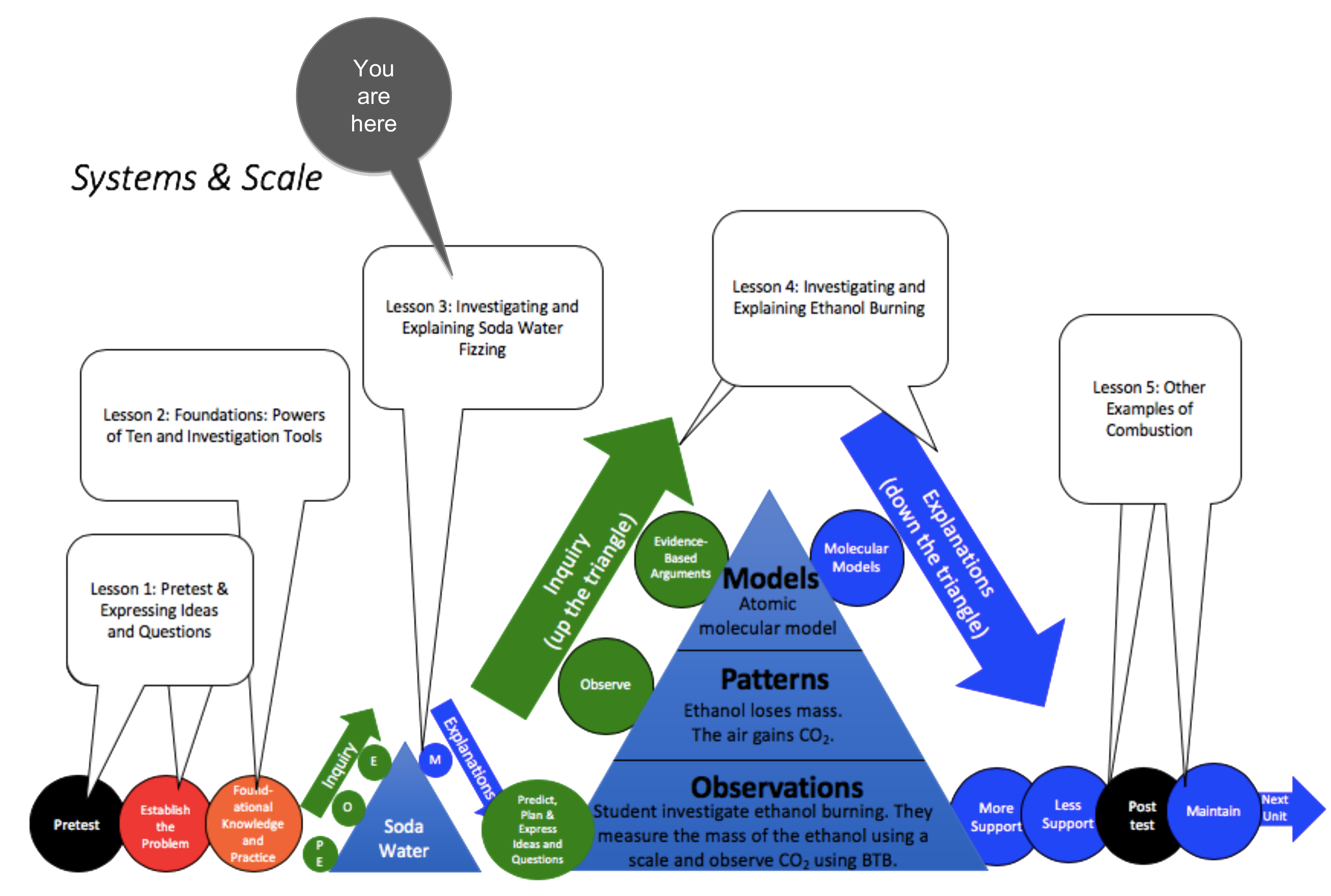
Target Performances
|
(Optional) Lesson 3 – Investigating and Explaining Soda Water Fizzing (students as investigators and explainers) |
|
|---|---|
|
Activity 3.1: Predictions and Planning for Soda Water Fizzing (20 min) |
Students develop hypotheses about how matter moves and changes when soda water loses its fizz and make predictions about how they can use their investigation tools—digital balances and BTB—to detect movements and changes in matter. |
|
Activity 3.2: Observing Soda Water Fizzing (30 min) |
Students record data about changes in mass and BTB when soda water fizzes and reach consensus about patterns in their data. |
|
Activity 3.3: Evidence-Based Arguments about Soda Water Fizzing (45 min) |
Students (a) use data from their investigations to develop evidence-based arguments about matter movements and matter changes when soda water fizzes, and (b) identify unanswered questions about matter movement and matter change that the data are insufficient to address. |
|
Activity 3.4: Molecular Models for Soda Water Fizzing (45 min) |
Students use molecular models to explain how carbon, oxygen, and hydrogen atoms are rearranged into new molecules during the decomposition of carbonic acid (the chemical change that happens when soda water fizzes). |
|
Activity 3.5: Explaining Soda Water Fizzing (40 min) |
Students explain how matter moves and changes when soda water loses its fizz (connecting macroscopic observations with atomic-molecular models and using the principle of conservation of matter). |
NGSS Performance Expectations
Middle School
- Structures and Properties of Matter. MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures.
- Chemical Reactions. MS-PS1-2. Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred.
- Chemical Reactions. MS-PS1-5. Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved.
High School
- Chemical Reactions. HS-PS1-7. Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
Talk and Writing
At this stage in the unit, students will complete the inquiry and application sequences for soda water fizzing—they go both up and down the triangle. This means that they will go through the Predictions Phase, the Observations Phase, the Evidence-Based Arguments Phase, and the Explanations Phase in one lesson. The tables below shows specific talk and writing goals for these phases of the unit.
|
Talk and Writing Goals for the Predictions Phase |
Teacher Talk Strategies That Support This Goal |
Curriculum Components That Support This Goal |
|---|---|---|
|
Treat this as elicitation and brainstorming (like the Expressing Ideas and Questions Phase), but with more directed questioning. |
Now that we have set up the investigation, we want to predict what we think will happen to matter and energy. |
Predictions and Planning Tool |
|
Elicit a range of student ideas. Press for details. Encourage students to examine, compare, and contrast their ideas with the ideas of other students. |
Who can add to that? What do you mean by _____? Say more. So I think you said _____. Is that right? Who has a different idea? How are those ideas similar/different? Who can rephrase ________’s idea? |
Investigation Video (first half) |
|
Encourage students to provide evidence that supports their predictions. . |
How do you know that? What have you seen in the world that makes you think that? |
|
|
Have students document their ideas to revisit later. |
Let’s record our ideas so we can come back to them and see how our ideas change. |
Predictions and Planning Tool |
|
Talk and Writing Goals for the Observations Phase |
Teacher Talk Strategies That Support This Goal |
Curriculum Components That Support This Goal |
|---|---|---|
|
Help students discuss data and identify patterns. |
What patterns do we see in our data? How do you know that is a pattern? What about ______ data. What does this mean? |
Class Results Poster Class Results Spreadsheet |
|
Encourage students to compare their own conclusions about the data and evidence with other groups and other classes. |
What about this number? What does this tell us? How is group A’s evidence different from Group B’s data? How do our class’s data differ from another classes’ data? |
Class Results Spreadsheet Class Results Poster Investigation Video (second half). |
|
Make connections between the observations and the data/evidence. |
It says here that our BTB turned colors. What does that mean? You recorded that your ethanol lost weight. What does that mean? |
|
|
Have students consider how their predictions and results compare. |
Let’s revisit our predictions. Who can explain the difference between our class predictions and our results? Who had predictions that were similar to our results? Has your explanation changed? How? |
|
Talk and Writing Goals for the Evidence-Based Arguments Phase |
Teacher Talk Strategies That Support This Goal |
Curriculum Components That Support This Goal |
|---|---|---|
|
Press for details. Encourage students to examine, compare, and contrast their ideas with the ideas of other students. |
Who can add to that argument? What do you mean by _____? Say more. So I think you said _____. Is that right? Who has a different argument? How are those arguments similar/different? Who can rephrase ________’s argument? |
Investigation Video (second half) |
|
Students provide evidence from the investigation (not just experiences in the world) to develop arguments. |
Does your argument include evidence from the investigation? What evidence is most important here?
What does this evidence tell us about what happened? How do you know that? |
Evidence-Based Arguments Tool Class Results Poster Class Results Spreadsheets Investigation Video (second half) Data from other classes |
|
Focus on how matter and energy were transformed at different scales. |
What does this evidence tell us about how matter is changing? What does this evidence tell us about how energy is changing? |
Evidence-Based Arguments Tool |
|
Revisit predictions and examine change in thinking. |
Let’s revisit our Predictions and see how our thinking changed now that we know what happened. |
Evidence-Based Arguments Tool Predictions and Planning Tool |
|
Encourage students to consider the questions they don’t have answers to. |
This investigation told us many things about what happen to matter and energy during ____. But what questions do we still have? |
|
Talk and Writing Goals for the Explanations Phase |
Teacher Talk Strategies That Support This Goal |
Curriculum Components That Support This Goal |
|---|---|---|
|
Examine student ideas and correct them when there are problems. It’s ok to give the answers away during this phase! Help students practice using precise |
Let’s think about what you just said: air molecules. What are air molecules? Are you talking about matter or energy? Remember: atoms can’t be created. So that matter must have come from somewhere. Where did it come from? Let’s look at the molecule poster again… is carbon an atom or a molecule? |
Molecule Poster |
|
Focus on making sure that explanations include multiple scales. |
The investigation gave us evidence for what was happening to matter and energy at a macroscopic sale. But what is happening at an atomic-molecular scale? What is happening to molecules and atoms? How does energy interact with atoms and molecules during chemical change? Why doesn’t the macroscopic investigation tell us the whole story? Let’s revisit our scale poster… what is happening to matter at the molecular scale? |
Molecular Models Molecular Modeling Worksheets Explanations Tool PPT Animation of chemical change Powers of Ten Poster |
|
Encourage students to recall the investigation. |
When did this chemical change happen during our investigation? How do we know that? What is our evidence? What were the macroscopic indicators that this chemical change took place? |
Evidence-Based Arguments Tool Investigation Video |
|
Elicit a range of student explanations. Press for details. Encourage students to examine, compare, and contrast their explanations. |
Who can add to that explanation? What do you mean by _____? Say more. So I think you said _____. Is that right? How are those explanations similar/different? Who can rephrase ________’s explanation? |
Explanations Tool |

 Download Lesson 3 Teacher's Guide PDF
Download Lesson 3 Teacher's Guide PDF