Transformations in Matter and Energy Carbon TIME is an NSF-funded partnership led by Michigan State University
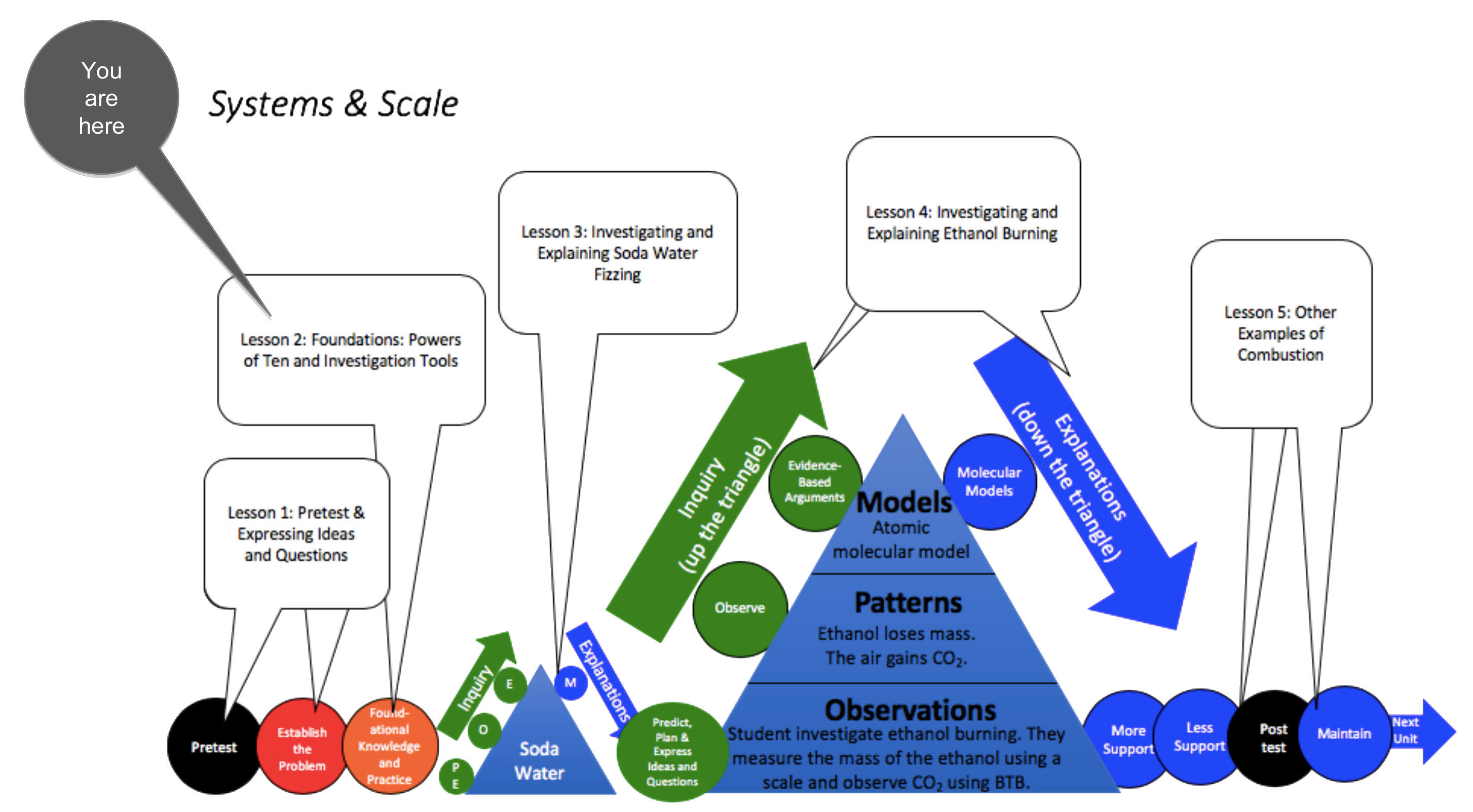
Lesson 2 - Powers of Ten and Investigation Tools
Students view the Powers of Ten video and discuss how all systems can be described at multiple scales. Then students learn that atoms last forever in physical and chemical changes. Finally, students practice using a digital balance and BTB, key tools in Carbon TIME units.
Guiding Question
What do materials look like at smaller and smaller scales?
Activities in this Lesson
Note: Activity 2.2 is optional depending on the academic level of your students. See the Systems and Scale Unit Read Me file for more information to consider when making this choice.
- Activity 2.1: Powers of Ten Video and Discussion (30 min)
- (Optional) Activity 2.2: From Big to Small (30 min)
- Activity 2.3: Zooming into Air (30 min)
- Activity 2.4: Atoms and Molecules Quiz and Discussion (20 min)
- Activity 2.5: Using a Digital Balance and BTB (30 min)
Unit Map
Target Performances
|
Lesson 2 – Foundations: Powers of Ten and Investigation Tools (students developing foundational knowledge and practice) |
|
|---|---|
|
Activity 2.1: Powers of Ten Video and Discussion (30 min) |
Students discuss how all systems can be analyzed by “zooming in” and “zooming out” through a hierarchy of systems at different scales. |
|
(Optional) Activity 2.2: From Big to Small (30 min) |
Students organize images to “zoom in” and “zoom out” of six different systems at four different scales: atomic-molecular, microscopic, macroscopic, and large scales. |
|
Activity 2.3: Zooming into Air (30 min) |
Students describe air at atomic-molecular, microscopic, macroscopic, and large scales, identifying specific molecules in air. |
|
Activity 2.4: Atoms and Molecules Quiz and Discussion (30 min) |
Students apply the principle of matter conservation to atoms and molecules in different phenomena. |
|
Activity 2.5: Using a Digital Balance and BTB (30 min) |
Students (a) practice using two key tools for investigation—digital balances and BTB—with accuracy and precision and (b) describe how they can use these tools to detect matter movement and matter change. |
NGSS Performance Expectations
Middle School
- Structures and Properties of Matter. MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures.
- Chemical Reactions. MS-PS1-2. Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred.
- Chemical Reactions. MS-PS1-5. Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved.
High School
- Chemical Reactions. HS-PS1-4. Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
- Chemical Reactions. HS-PS1-7. Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
At this stage in the unit, the students will be learning Foundational Knowledge and Practice that is important for the rest of the unit. The table below shows specific talk and writing goals for this phase of the unit.
|
Talk and Writing Goals for the Foundations Phase |
Teacher Talk Strategies That Support This Goal |
Curriculum Components That Support This Goal |
|---|---|---|
|
Treat this as background information. |
We want to talk about a few basic practices and some basic knowledge to prepare us for the unit. |
|
|
Listen for student ideas about matter and energy at different scales, and attend to wrong ideas. |
What is happening to matter and energy at ______ scale? Who can explain? Are you in the macroscopic scale or the atomic-molecular scale? Who can explain that at a different scale? |
The PPT that “Zooms into” the macroscopic subjects of the unit: a leaf, a potato, air, fossil fuels, etc. |
|
Examine student ideas and correct them when there are problems. It’s ok to give the answers away during this phase! Help students practice using precise language to describe matter and energy at different scales. |
Let’s think about what you just said: air molecules. What are air molecules? Are you talking about matter or energy? Remember: atoms can’t be created. So that matter must have come from somewhere. Where did it come from? Let’s look at the molecule poster again… is carbon an atom or a molecule? Let’s revisit our scale poster… what is happening to matter at a macroscopic scale? |
Powers of Ten Video Powers of Ten Poster Molecule Poster Three Questions Poster
|
|
Grade student ideas. |
|
There is a quiz during this phase of the unit to help you decide if your students are ready to move on. |

 Download Lesson 2 PDF Teacher's Guide
Download Lesson 2 PDF Teacher's Guide