Transformations in Matter and Energy Carbon TIME is an NSF-funded partnership led by Michigan State University
Plants | Pre-Lesson 1 - Investigation Setup
Students set up an investigation in preparation for the Plants Unit. Plants will be ready to harvest about four weeks later.
Guiding Question
How does a plant gain mass?
Activities in this Lesson
Note: There are two different pathways to choose from in the Pre-Lesson. Please see the Plants Unit Read Me Document, Student Challenges and Teacher Choices in the Plants Unit document and/or the Background Information section below for clarification in making this instructional decision.
 Gel Protocol (2-turtle)
Gel Protocol (2-turtle)
- Pre-Activity 0.1GL: Keeping Track of Water in Solids and Liquids (60 min + overnight or several days)
- Pre-Activity 0.2GL: Plant Growth Investigation Setup (45-60 min over one or two days)
 Paper Towel Protocol (1-turtle)
Paper Towel Protocol (1-turtle)
- Pre-Activity 0.2PT: Plants Growth Investigation Setup (45-60 min)
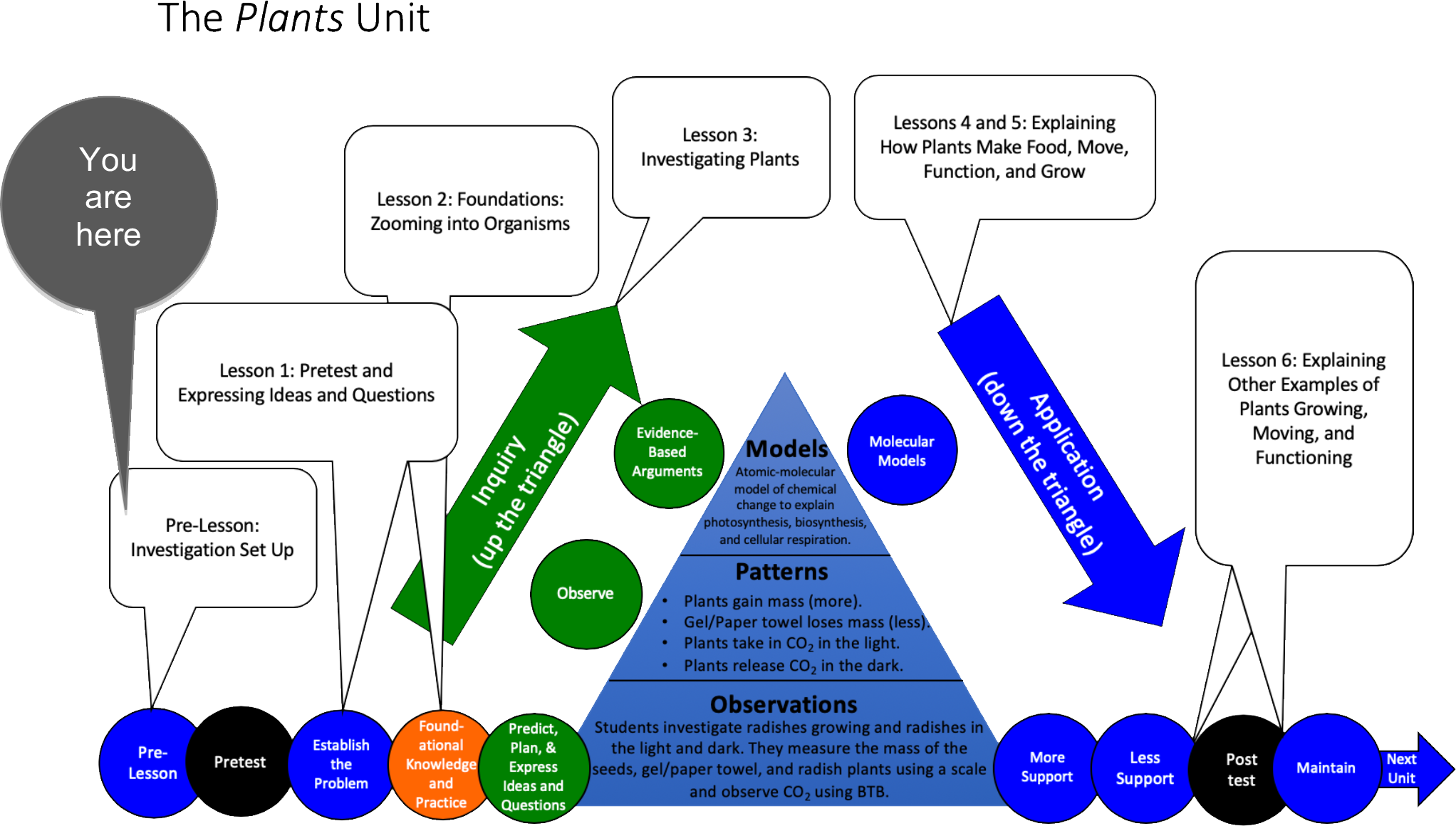
Unit Map
Target Performances
|
Activity |
Target Performance |
|
Pre-Lesson 1: Investigation Setup |
|
|---|---|
|
Pre-Activity 0.2GL: Keeping Track of Solids in Mixtures |
Students will distinguish between solid mass (or dry mass) and total mass of materials consisting of water mixed with solid materials and use measurement techniques to determine the mass of solids in the mixtures. |
|
Pre-Activity 0.2GL: Plant Growth Investigation Setup |
Students will make initial measurements of the dry mass of radish seeds and growth media and start plants growing. |
|
Pre-Activity 0.2PT: Plant Growth Investigation Setup |
Students will make initial measurements of the dry mass of radish seeds and growth media and start plants growing. |
NGSS Performance Expectations
Middle school
- MS. Matter and Energy in Organisms and Ecosystems. MS-LS1-6. Construct a scientific explanation based on evidence for the role of photosynthesis in the cycling of matter and flow of energy into and out of organisms.
High school
- HS. Chemical Reactions. HS-PS1-7. Use mathematical representations to support that claim that atoms, and therefore mass, are conserved during a chemical reaction.

 Download PDF of Pre-Lesson Lesson 1 Teacher's Guide
Download PDF of Pre-Lesson Lesson 1 Teacher's Guide