Transformations in Matter and Energy Carbon TIME is an NSF-funded partnership led by Michigan State University
Decomposers | Lesson 2 - Foundations: Zooming into Decomposers
Students “zoom into” plants, animals, and decomposers to learn about structures and functions that they all share:
- All organisms are made of cells.
- All the functions of organisms are done by their cells.
- All cells are made of molecules, including large organic molecules (fats, carbohydrates, and proteins).
- All functions of cells involve moving and changing molecules.
Guiding Question
What makes up our food?
Activities in this Lesson
Note: Activities 2.1, 2.2, and 2.3 are repeating activities: They are the same in the three organism-scale units: Animals, Plants, and Decomposers. Activity 2.4 focuses specifically on decomposers. Teach Activities 2.1, 2.2 and 2.3 during the first of the organism-scale units you teach (Animals, Plants or Decomposers). These activities can be skipped when you teach subsequent organism-scale units. Activity 2.4 is different for each unit though and should be taught each time.
-
 Activity 2.1: Zooming into Plants, Animals, and Decomposers (40 min)
Activity 2.1: Zooming into Plants, Animals, and Decomposers (40 min) -
 Activity 2.2: Molecules Cells Are Made of (45 min)
Activity 2.2: Molecules Cells Are Made of (45 min) -
 Activity 2.3: Molecules in Cells Quiz (20 min)
Activity 2.3: Molecules in Cells Quiz (20 min) - Activity 2.4: Questions about Decomposers (20 min)
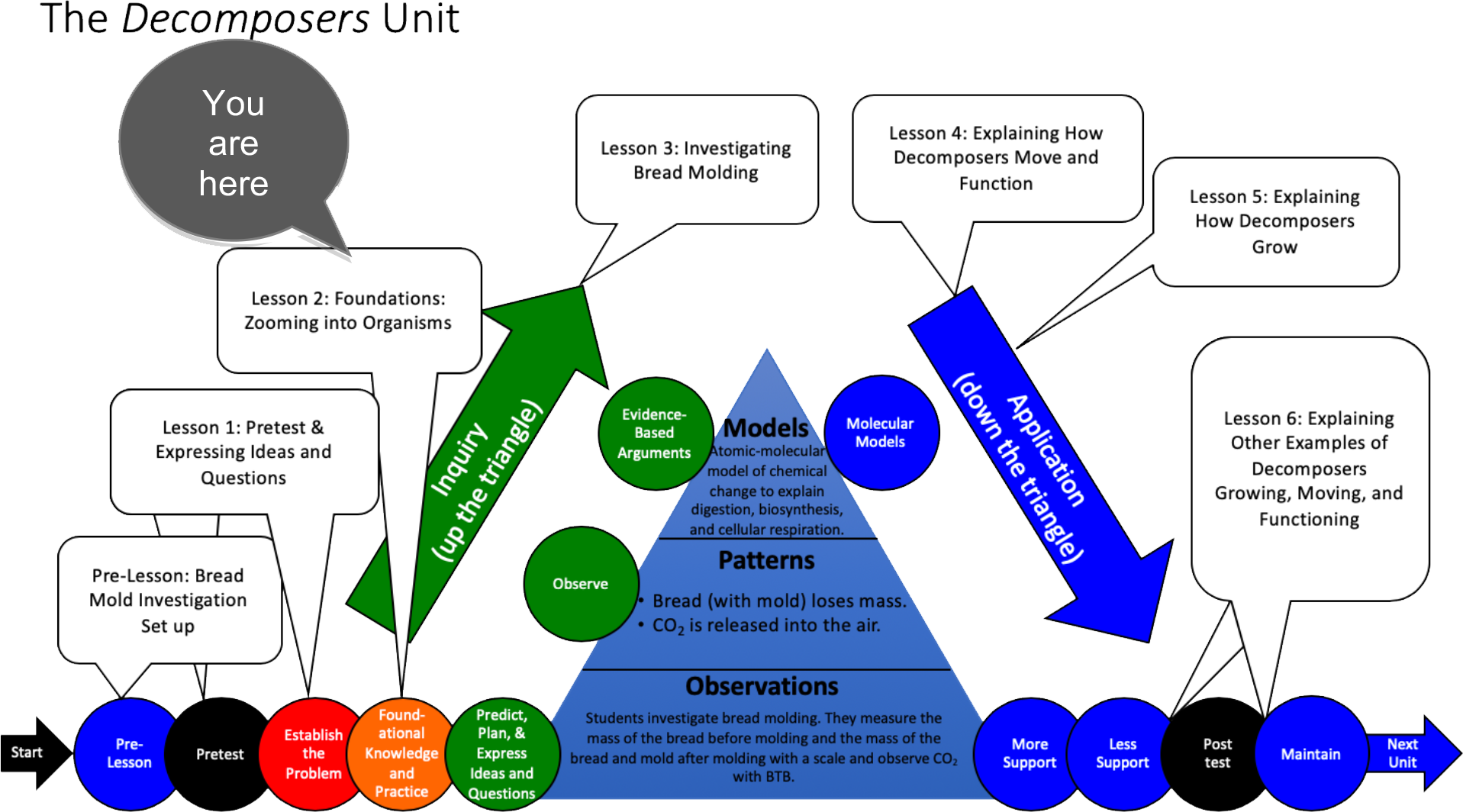
Unit Map
Target Performances
|
Activity |
Target Performance |
|
Lesson 2 – Foundations: Zooming into Organisms (students developing foundational knowledge and practice) |
|
|---|---|
|
Activity 2.1: Zooming into Plants, Animals, and Decomposers |
Students “zoom in” to animals, plants, and decomposers, describing how all of these organisms are made of cells with special structures and functions. |
|
Activity 2.2: Molecules Cells Are Made of |
Students use food labels to describe molecules in animal, plant, and decomposer cells: large organic molecules (carbohydrates, proteins, and fats), as well as water, vitamins, and minerals. |
|
Activity 2.3: Molecules in Cells Quiz |
Students complete a quiz to assess their understanding of the molecules in cells and how to identify which molecules store chemical energy. |
|
Activity 2.4: Questions about Decomposers |
Students describe structures and functions that all decomposers share and pose questions about molding bread to prepare for their upcoming investigation. |
NGSS Performance Expectations
High School
- Structure and Function. HS-LS1-2. Develop and use a model to illustrate the hierarchical organization of interacting systems that provide specific functions within multicellular organisms.
- From Molecules to Organisms: Structures and Processes. HS-LS1-6. Construct and revise an explanation based on evidence for how
carbon, hydrogen, and oxygen from sugar molecules may combine with other elements
to form
amino acids and/or other large carbon-based molecules.
Middle School
- Matter and its Interactions. MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures.
- Structure, Function, and Information Processing. MS-LS1-3. Use argument supported by evidence for how the body is a system of interacting subsystems composed of groups of cells.
Talk and Writing
At this stage in the unit, the students will be learning Foundational Knowledge and Practice that is important for the rest of the unit. The table below shows specific talk and writing goals for this phase of the unit.
| Talk and Writing Goals for the Foundations Phase | Teacher Talk Strategies That Support This Goal | Curriculum Components That Support This Goal |
|---|---|---|
|
Treat this as background information. |
We want to talk about a few basic practices and some basic knowledge to prepare us for the unit. |
|
|
Listen for student ideas about matter and energy at different scales and attend to wrong ideas. |
What is happening to matter and energy at ______ scale? Who can explain? Are you in the macroscopic scale or the atomic-molecular scale? Who can explain that at a different scale? |
The PPT that “Zooms into” the macroscopic subjects of the unit: a leaf, a potato, air, fossil fuels, etc. |
|
Examine student ideas and correct them when there are problems. It’s ok to give the answers away during this phase! Help students practice using precise |
Let’s think about what you just said: food molecules. What are food molecules? Are you talking about matter or energy? Remember: atoms can’t be created. So that matter must have come from somewhere. Where did it come from? Let’s look at the molecule poster again… is carbon an atom or a molecule? Let’s revisit our scale poster… what is happening to matter at a macroscopic scale? |
Powers of Ten Poster Molecule Poster Three Questions Poster |
|
Grade student ideas. |
There is a quiz during this phase of the unit to help you decide if your students are ready to move on. |

 Download PDF of Lesson 2 Teacher's Guide
Download PDF of Lesson 2 Teacher's Guide