Transformations in Matter and Energy Carbon TIME is an NSF-funded partnership led by Michigan State University
Decomposers | Lesson 6 - Explaining Other Examples of Decomposers...
Students do optional readings and activities about different types of decomposers. Then they practice explaining other examples of decomposers growing, moving and functioning and take the unit posttest.
Guiding Question
How do other decomposers grow, move, and function?
Activities in this Lesson
- (Optional) Activity 6.1: Exploring Different Kinds of Decomposers (varies)
- Activity 6.2: Explaining Other Examples of Decomposers Growing, Moving, and Functioning (50 min)
- Activity 6.3: Comparing Decomposers, Plants, and Animals (50 min)
- Activity 6.4: Functions of All Decomposers (50 min)
- Activity 6.5: Decomposers Unit Posttest (40 min)
Unit Map
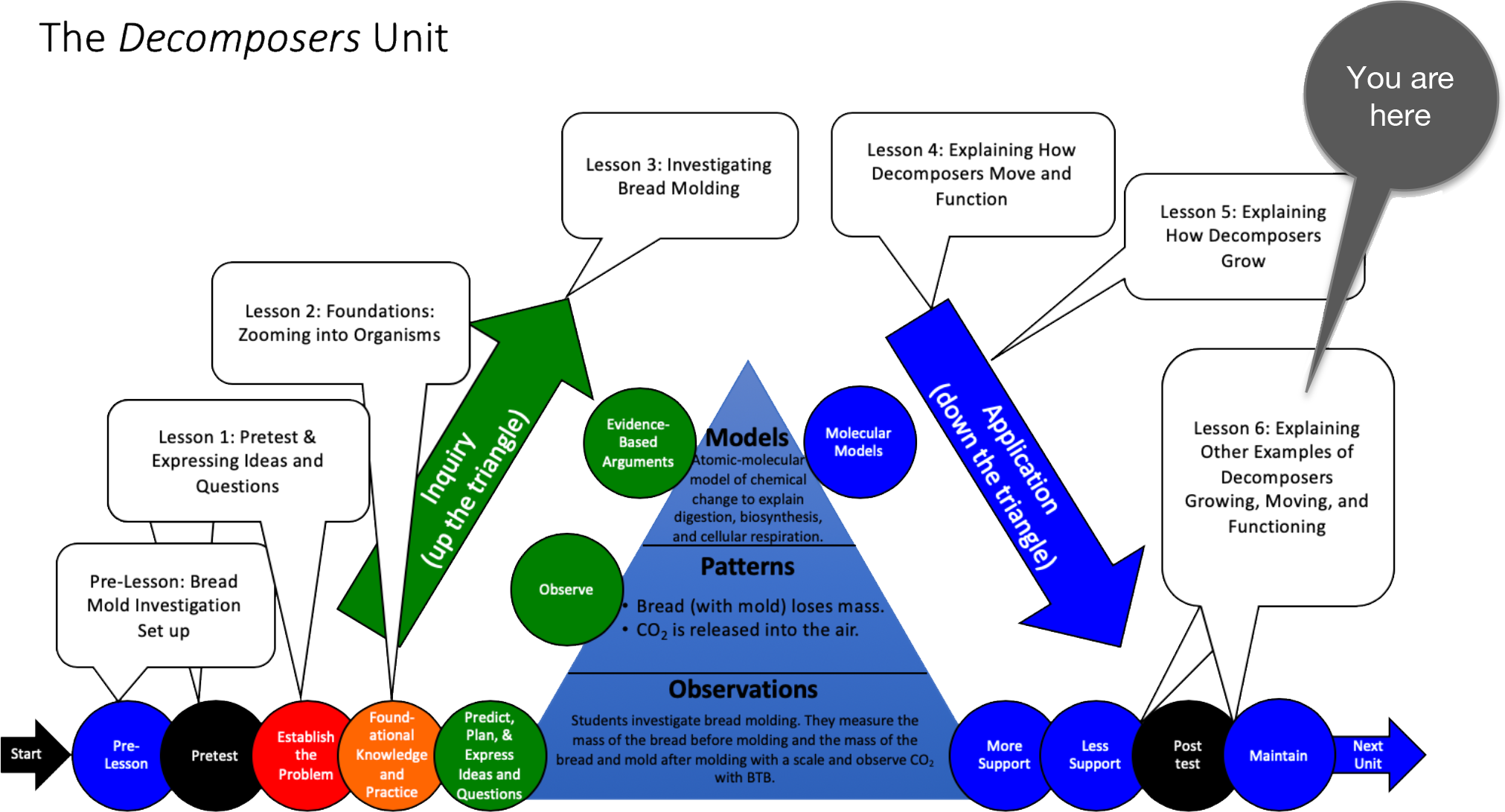
Target Performances
|
Activity |
Target Performance |
|
Lesson 6 – Explaining Other Examples of Decomposers Growing, Moving, and Functioning (students as explainers) |
|
|---|---|
|
(Optional) Activity 6.1: Exploring Different Kinds of Decomposers |
Students explain how matter and energy move and change in other phenomena involving decomposers, included aerobic and anaerobic bacteria, fermentation, spontaneous combustion of hay, and decomposition in forests. |
|
Activity 6.2: Explaining Other Examples of Decomposers Growing, Moving, and Functioning |
Students develop integrated accounts of how other fungi (bracket fungi, bread mold, mycorrhizal fungi) grow and function through the processes of digestion, cellular respiration, and biosynthesis. |
|
Activity 6.3: Comparing Decomposers, Plants, and Animals |
Students compare how matter moves and changes and how energy changes in decomposers, plants, and animals. |
|
Activity 6.4: Functions of All Decomposers |
Students develop integrated accounts of how all aerobic decomposers grow and function through the processes of digestion, cellular respiration, and biosynthesis. |
|
Activity 6.5: Decomposers Unit Posttest |
Students show their end-of unit proficiencies for the overall unit goal: Questioning, investigating, and explaining how decomposers move and change matter and energy as they live and grow. |
NGSS Performance Expectations
High School
- Chemical Reactions. HS-PS1-4. Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends on the changes in total bond energy.
- Chemical Reactions. HS-PS1-7. Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
- From Molecules to Organisms: Structures and Processes. HS-LS1-2. Develop and use a model to illustrate the hierarchical organization of interacting systems that provide specific functions within multicellular organisms.
- Matter and Energy in Organisms and Ecosystems. HS-LS1-6. Construct and revise an explanation based on evidence for how carbon, hydrogen, and oxygen from sugar molecules may combine with other elements to form amino acids and/or other large carbon-based molecules.
- Matter and Energy in Organisms and Ecosystems. HS-LS1-7. Use a model to illustrate that cellular respiration is a chemical process whereby the bonds of food molecules and oxygen molecules are broken and the bonds in new compounds are formed resulting in a net transfer of energy.
- Matter and Energy in Organisms and Ecosystems. HS-LS2-3. Construct and revise an explanation based on evidence for the cycling of matter and flow of energy in aerobic and anaerobic conditions.
Middle School
- Structure and Properties of Matter. MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures.
- Chemical Reactions. MS-PS1-5. Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved.
- From Molecules to Organisms: Structures and Processes. MS-LS1-3. Use argument supported by evidence for how the body is a system of interacting subsystems composed of groups of cells.
- Matter and Energy in Organisms and Ecosystems. MS-LS1-7. Develop a model to describe how food is rearranged through chemical reactions forming new molecules that support growth and/or release energy as this matter moves through an organism.
- Matter and Energy in Organisms and Ecosystems. MS-LS2-3. Develop a model to describe the cycling of matter and flow of energy among living and nonliving parts of an ecosystem.
Talk and Writing
This lesson of the unit represents the fading portion of the Explanations Phase. This means that students are expected to develop explanations for carbon-transforming processes they studied in this unit in new and novel contexts. The table below shows specific talk and writing goals for the Explanations Phase of the unit.
| Talk and Writing Goals for the Explanations Phase | Teacher Talk Strategies That Support This Goal | Curriculum Components That Support This Goal |
|---|---|---|
|
Examine student ideas and correct them when there are problems. It’s ok to give the answers away during this phase! Help students practice using precise language to describe matter and energy. |
Let’s think about what you just said: air molecules. What are air molecules? Are you talking about matter or energy? Remember: atoms can’t be created. So that matter must have come from somewhere. Where did it come from? Let’s look at the molecule poster again… is carbon an atom or a molecule? |
Molecule Poster Three Questions Poster
|
|
Focus on making sure that explanations include multiple scales. |
The investigation gave us evidence for what was happening to matter and energy at a macroscopic sale. But what is happening at an atomic-molecular scale? What is happening to molecules and atoms? How does energy interact with atoms and molecules during chemical change? Why doesn’t the macroscopic investigation tell us the whole story? Let’s revisit our scale poster… what is happening to matter at the molecular scale? |
Molecular Models Molecular Modeling Worksheets Explanation Tools PPT Animation of chemical change Powers of Ten Poster |
|
Encourage students to recall the investigation. |
When did this chemical change happen during our investigation? How do we know that? What is our evidence? What were the macroscopic indicators that this chemical change took place? |
Evidence-Based Arguments Tool Investigation Video |
|
Elicit a range of student explanations. Press for details. Encourage students to examine, compare, and contrast their explanations with others’. |
Who can add to that explanation? What do you mean by _____? Say more. So, I think you said _____. Is that right? Who has a different explanation? How are those explanations similar/different? Who can rephrase ________’s explanation? |
Explanation Tools |

 Download PDF of Lesson 6 Teacher's Guide
Download PDF of Lesson 6 Teacher's Guide